New drug for growth hormone deficiency in children!
March 5, 2019 / Bio Valley BIOON / -- Danish biopharmaceutical company Ascendis Pharma recently announced the weekly topping of the HeiGHt phase III clinical trial of TransCon growth hormone (hGH) in children with growth hormone deficiency (GHD) data.
The study was a randomized, open-label, positive drug-controlled study comparing weekly TransCon hGH with once-daily hGH (Genotropin). The results showed that the study reached the primary endpoint: At 52 weeks, TransCon hGH was not inferior to daily hGH and better than daily hGH in terms of annualized height rate (AHV, unit: cm/year). The specific data was that the covariance analysis (ANCOVA) intention-to-treat group was used. The TransCon hGH treatment group had an AHV of 11.2 cm/year and a daily hGH of 10.3 cm/year (95% CI: 0.22-1.50, p=0.0088).
At each visit, the AHV of the TransCon hGH treatment group was higher than the daily hGH treatment group, and the treatment difference was statistically significant from the 26th week (including the 26th week). The proportion of patients with poor response (AHV < 8.0 cm/year) in the TransCon hGH-treated group and the daily hGH-treated group was 4% and 11%, respectively. All sensitivity analyses completed in the study support the main results, indicating the robustness of these results.
In this study, TransCon hGH was generally safe, well tolerated, and the adverse events were consistent with the type and frequency observed in the daily hGH treatment group, and the trial groups were comparable. No serious adverse events associated with the study drug were found in either group, and one serious adverse event was observed in each treatment group (1.0% in the TransCon hGH group and 1.8% in the daily hGH group), all of which were not related to the study drug. None of the two groups found adverse events leading to treatment discontinuation of the study drug.
Jan Mikkelsen, President and CEO of Ascendis Pharmaceuticals, said, "The results of the heiGHt study released today are a potential breakthrough for patients and future growth hormone deficiency treatment programs. The results show that TransCon-hGH has superior efficacy and Daily hGH is fairly safe and tolerant. We believe these results provide validation for our TransCon technology platform, which is the basis of our endocrine pipeline and has potential applications in other therapeutic areas."
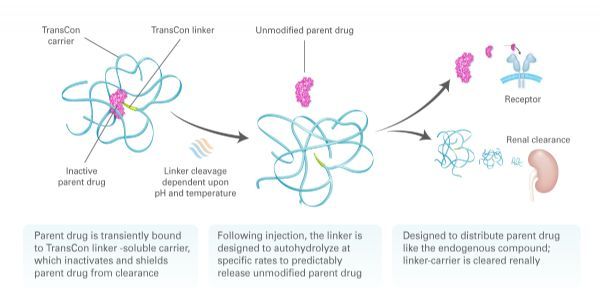
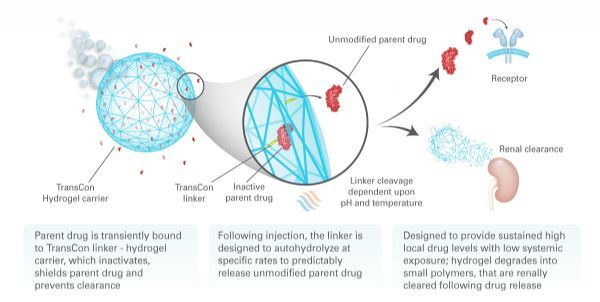
TransCon means "instantaneous bonding." Ascendis's proprietary TransCon platform is an innovative technology designed to create new therapies that optimize treatment outcomes, including efficacy, safety and frequency of dosing. The TransCon molecule has three components: an unmodified parent drug, an inert carrier that protects it, and a linker that temporarily binds to both. When combined, the carrier is inactivated and the parent drug is protected from clearance. When injected into the body, physiological pH and temperature conditions begin to release the unmodified active parent drug in a predictable release manner. Because the parent drug is unmodified, its original mode of action should remain the same. TransCon technology is widely used in multiple therapeutic areas of proteins, peptides or small molecules and can be used systemically or locally.
Child growth hormone deficiency (GHD) is a serious and rare disease caused by the inability of the pituitary gland to produce sufficient growth hormone. Children with GHD are not only short stature, but also experience metabolic abnormalities, psychosocial challenges, cognitive deficits, and poor quality of life.
For decades, GHD's standard of care has been to subcutaneously inject hGH once a day, which improves growth and metabolism. For caregivers and patients, the daily injection has a high therapeutic burden, which leads to poor compliance and lower overall treatment outcomes.












